Weibold Academy: Unlocking economic potential of rCB demineralization
Weibold Academy article series discusses periodically the practical developments and scientific research findings in the end-of-life tire (ELT) recycling and pyrolysis industry.
These articles are reviews by Claus Lamer – the senior pyrolysis consultant at Weibold. One of the goals of the review is to give entrepreneurs in this industry, project initiators, investors and the public, a better insight into a rapidly growing circular economy. At the same time, this article series should also be a stimulus for discussion.
For the sake of completeness, we would like to emphasize that these articles are no legal advice from Weibold or the author. For legally binding statements, please refer to the responsible authorities and specialist lawyers.
Introduction
End-of-life tires represent a significant global waste problem. Their robust structural and chemical properties pose challenges for traditional recycling methods. With 1.5 billion tires manufactured annually, the environmental impact is substantial, with each tire contributing approximately 300 kg of CO2 emissions. The increase in tire production, driven by the rise of electric vehicles, underscores the urgency for effective recycling strategies.
Waste tires are not only difficult to dispose of but also pose environmental hazards when dumped or incinerated. Traditional disposal methods like open dumping and incineration lead to issues such as fire hazards, mosquito breeding grounds, and the release of harmful pollutants.
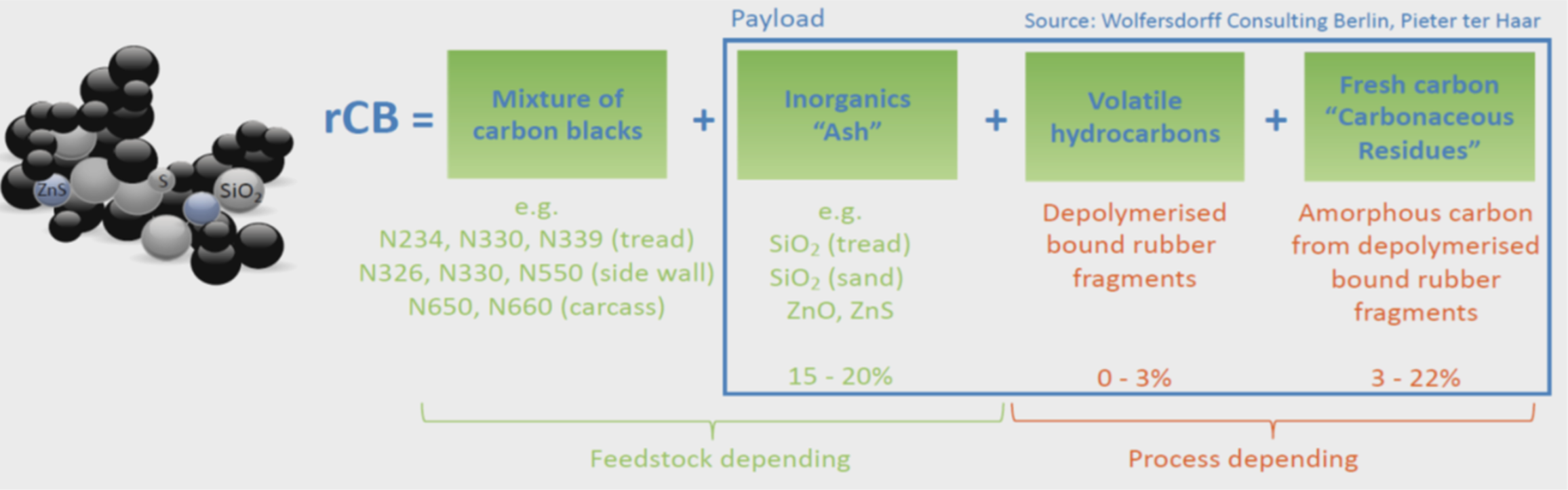
Pyrolysis, the process of decomposing organic material at high temperatures in an oxygen-free environment, emerges as a promising method for tire recycling. This process yields char, oil, and gas, with the char containing valuable carbon black. This carbon black, when recovered and purified, is known as Recovered Carbon Black (rCB). rCB holds potential as a substitute for virgin carbon black in various polymer products, including new tires, thereby promoting closed-loop recycling.
However, the performance of rCB is hindered by contaminants such as volatile matter (volatile hydrocarbons, “VOC”) (organic) and inorganic residues (often referred to as “ash”). The presence of metals like zinc and iron, along with other elements, compromises the material's properties, limiting its applications. A new study points to techniques that have the potential to profitably increase the value of rCB by adding an extra step in the rCB finishing suite of tire pyrolysis operations.
Studying the impact and economic viability of demineralization
To address this, a study at the Imperial College London aims to assess the impact of demineralization on the physiochemical properties of rCB and investigate the potential for recovering valuable metals from demineralized rCB.
This study by Alex Bowles and colleagues involved the pyrolysis of tire granulates to produce rCB, followed by demineralization using hydrothermal washing and hydrochloric acid (HCl) treatments at different concentrations. The effectiveness of these treatments was evaluated based on the reduction of ash content and improvement in the carbon content of rCB.
The pyrolysis process involves heating tire granulates in an oxygen-free environment to break down the complex polymers in the rubber. This generates three main products: pyrolytic oil, gases, and char. The char is rich in carbon black, which can be further processed to produce rCB.
The pyrolysis was conducted at a temperature of 550°C using a batch rotary kiln under a continuous flow of nitrogen. This method ensures that the tire rubber is fully decomposed, yielding a char with high carbon content and minimal volatile matter. The char obtained from this process is referred to as raw-rCB.
Demineralization techniques
Demineralization was performed using two primary methods: hydrothermal washing and acid washing to improve the quality of rCB.
- Hydrothermal Washing: This method involves washing the raw-rCB with deionized water using a Soxhlet extraction system. The purpose is to remove soluble inorganic compounds and reduce the ash content.
- Acid Washing: Raw-rCB was treated with hydrochloric acid at 0.1M and 5M concentrations. The acid washing was conducted at room temperature with constant stirring to ensure uniform treatment. The treated samples were filtered and washed with de-ionized water until the filtrate reached a neutral pH.
Analysis of demineralized rCB
The demineralized rCB samples were subjected to various analytical techniques to assess their composition and properties:
- Proximate Analysis: Determined the ash content and carbon content of the rCB samples.
- Elemental Analysis: Measured the levels of carbon, hydrogen, nitrogen, sulfur, and oxygen in the samples.
- Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES): Quantified the metallic elements present in the rCB.
- Fourier Transform Infra-Red Spectroscopy (FTIR): Identified the functional groups present on the surface of the rCB.
- X-ray Diffraction (XRD): Analyzed the crystalline phases in the rCB samples.
- Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM): Provided detailed images of the rCB structure and the distribution of inorganic elements.
Findings and discussion
Ash content reduction
The initial ash content of Raw-rCB was 16.1%, which significantly decreased after demineralization. Hydrothermal washing reduced the ash content to 13.13%, while 0.1M HCl and 5M HCl treatments brought it down to 10.5% and 5.78%, respectively. This reduction is attributed to the removal of metals and other inorganic components, enhancing the rCB's properties.
Improvement in carbon content
The carbon content of rCB increased from 80.05% in Raw-rCB to 88.4% after 5M HCl treatment. This increase indicates the successful removal of contaminants, making the rCB more suitable for use in polymer matrices.
Metal removal efficiency
The effectiveness of demineralization in removing metallic elements was assessed through ICP-OES analysis. The results showed a significant reduction in the levels of zinc, iron, and other metals. For instance, the zinc content decreased from 46.17 g/kg in Raw-rCB to 0.21 g/kg after 5M HCl treatment, demonstrating the high efficiency of acid washing.
Impact on physical structure
SEM and TEM analyses revealed changes in the physical structure of rCB after demineralization. The removal of inorganic elements resulted in a more homogenous and porous structure, improving the material's dispersibility and interaction with polymer matrices.
Surface chemistry
FTIR analysis indicated changes in the surface functional groups of rCB following demineralization. The removal of sulfur and metal-associated groups was evident, contributing to a more uniform surface chemistry that enhances the bonding between rCB and polymers.
The economic potential of rCB metal recovery
rCB demetallization and desilication
The economic feasibility of rCB (Recycled Carbon Black) demineralization at an industrial scale remains unstudied. Selective demineralization using solvents that do not dissolve silicates suggests that rCB demineralization can be sub-categorized into demetallization, desulfurization, and desilication, targeting the recovery of metals, sulfur, and precipitated silica respectively. However, economically recovering the heterogeneous silica structures is improbable due to the high cost and hazardous nature of desilication agents like HF/HNO3 compared to demetallization agents such as 5 M HCl. For desilicated rCB to be profitable, it must significantly outperform demetallized rCB in rubber applications. Alternative, cost-effective measures such as controlling tire pyrolysis feedstock can minimize and homogenize silica content.
Value of rCB metallic components
The economic value of rCB's metallic content is estimated at approximately $155 per ton of rCB, equating to about $57 of char-derived metals per ton of steel-free tire granulate. The majority of this value comes from zinc (84%) and cobalt (12%). For a tire pyrolysis facility processing 100,000 tons per year, the metallic content could be valued at around $5.7 million annually, predominantly from zinc.
Solvent costs
Commercial pyrolysis and demineralization costs include solvent and metal extraction expenses. Based on previous studies, approximately 3.94 tons of 37% HCl is needed to demineralize 1 ton of rCB. The rCB demineralization process consumed around 170 kg of 37% HCl (cHCl) per ton of rCB, costing about $25 per ton. Effective HCl regeneration strategies are crucial, as the residual HCl (rHCl) cost is estimated at $567 per ton of rCB. Electrowinning (electrolysis) is also a promising method for metal extraction and solvent recovery, with a predicted operational expense of less than $50 per ton of demineralized rCB.
Conclusion
Effective demineralization and metal recovery from rCB have significant economic potential, particularly through optimizing solvent use and leveraging advanced extraction methods like electrowinning (electrolysis). While reduced ash content is not a requirement for many applications for rCB, the additional OPEX of $50 to $60 per ton of demineralized rCB could achieve a significantly higher rCB material value for specific applications. The study therefore highlights an opportunity for tire pyrolysis operators to institute an additional process step to increase the value of their rCB output stream worth exploring.
Authors' Note & Source
This article is based on research conducted by Alex J. Bowles, Amy L. Wilson, and Geoffrey D. Fowler from the Department of Civil & Environmental Engineering at Imperial College London. The study underscores the importance of innovative recycling methods in addressing global waste challenges and highlights the potential of recovered carbon black demineralization in tire recycling.
For more detailed information, please refer to the full study available on Elsevier's website: Bowles, A. J., Wilson, A. L., Fowler, G. D. (2023). Synergistic benefits of recovered carbon black demineralization for tire recycling. Resources, Conservation & Recycling, 198, 107124. Available online: Elsevier. This study was published under the open-access CC BY license.
Weibold is an international consulting company specializing exclusively in end-of-life tire recycling and pyrolysis. Since 1999, we have helped companies grow and build profitable businesses.









