Weibold Academy: "Waste4GreenFuture" by chemical recycling of tires
Weibold Academy article series discusses periodically the practical developments and scientific research findings in the end-of-life tire (ELT) recycling and pyrolysis industry.
This article is a review by Claus Lamer – the senior pyrolysis consultant at Weibold. One of the goals of this review is to give entrepreneurs in this industry, project initiators, investors and the public, a better insight into a rapidly growing circular economy. At the same time, this article series should also be a stimulus for discussion.
For the sake of completeness, we would like to emphasize that these articles are no legal advice from Weibold or the author. For legally binding statements, please refer to the responsible authorities and / or specialist lawyers.
Introduction
Chemical recycling is an emerging theme in waste management which has the potential to contribute to a low carbon, resource efficient and sustainable economy. In enabling the use of carbonaceous waste as secondary carbon feedstock to replace fossil resources to produce organic chemicals, it could support efforts to transit carbon intensive industries from the linear to a circular carbon economy.
A sustainable society with climate-neutral processes requires significant adjustments in the value chains, which are only possible through innovations. In the “Waste4Future” lighthouse project, seven institutions of the Fraunhofer-Gesellschaft (Germany) are pooling their expertise to develop new solutions for this goal, from the raw material basis to material flows and process engineering to the end of a product’s life cycle. They aim to increase energy and resource efficiency when using plastics and thus pave the way for a chemical industry that requires fewer fossil raw materials and causes fewer emissions.
An exploratory case study approach recently assessed the perception of chemical recycling and its perceived role in the transformation of Germany’s chemical and waste management industries. The outcome revealed policy and regulatory needs which will have to be addressed to successfully implement chemical recycling as a complementary building block of sustainable waste management.
Tremendous increase in waste
Globally, increasing amounts of natural resources are required as raw materials to meet the demands of a growing population, economic developments, rising living standards, decarbonization measures and technological breakthroughs. Parallel to the increasing resource consumption is the production of a growing amount of waste. Between 2012 and 2025, annual global solid waste production is likely to increase from 1.3 billion tons to 2.2 billion tons. A further increase of up to 3.4 billion tons of waste per annum by 2050 is predicted if business-as-usual continues. Consequently, waste disposal is becoming a critical challenge for many societies.
A one-way street
In today’s linear economy, waste is disposed predominantly via landfill and waste incineration. The former is associated with challenges such as loss of land for value-added developments, shortage of available landfill sites, emissions of landfill gases, environmental contamination and harms to health and safety. Problems faced by the later range from low efficiency utilization of waste, significant climate-relevant and polluting emissions, air pollution through particulate matter, safe disposal of toxic fly ash to public resistance. Besides these challenges, both disposal pathways also represent a loss of carbon resources which could potentially be recirculated as secondary carbon feedstock for the organic chemical industry, and thus contribute to resource conservation by reducing the chemical industry’s dependency on fossil raw materials.
Increasing circularity
To support our society’s sustainability transformation towards a low carbon and resource-efficient economy, the waste management and chemical sectors are thus anticipated to play important roles, especially in the transition towards a circular carbon economy via recycling of carbonaceous waste. To date, efforts have predominantly focused on mechanical recycling to recycle waste materials into ‘‘new” (secondary) raw materials without changing the basic chemical structure of the source material. In recent years, chemical recycling (CR) – with its potential to reduce environmental impacts compared to waste incineration and landfilling via enabling circularity of waste – has been gaining increasing global attention as a possible complement to mechanical recycling for carbonaceous waste (i.e., plastic, rubber) which cannot be mechanically recycled.
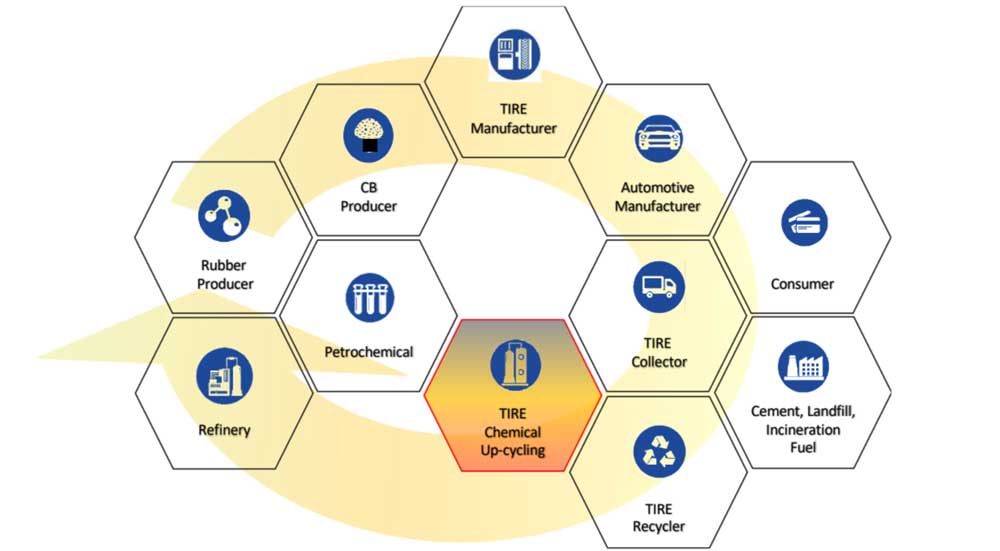
Chemical recycling - The missing link
Chemical recycling is used to describe technologies which convert carbonaceous waste (i.e., plastic, rubber) chemically - generally under high temperatures and if necessary, under high pressure conditions and/or with the addition of solvents and reacting gases - into valuable base chemicals which can then be used as feedstock for the chemical industry. Unlike mechanical recycling, chemical recycling changes the basic chemical structure of the source material. Therefore, chemical recycling operations are considered as chemical manufacturing and not waste management because these processes generate valuable marketable products for commerce just like any chemical manufacturing facility (BASF USA, 2021).* In the USA 14 states already set important precedents enacting laws that legally define chemical recycling as a manufacturing process.*
In the last years, the applicability of chemical recycling processes - specially to promote circularity of plastic waste to resolve the global plastic crisis - has been driving chemical recycling research, socio-political discussions as well as global developments.
While the main political emphasis is (still) on reducing, reusing and mechanical recycling of plastic waste, science and industry are increasingly highlighting the need to establish the role of chemical recycling before waste incineration to complement mechanical recycling, and to extend the focus of chemical recycling from plastic waste to other carbonaceous waste (i.e., waste rubber and end-of-life tires).
Chemical recycling processes
Chemical recycling processes can be generally categorized into solvolysis, pyrolysis and gasification. During solvolysis, solvents are used to dissolve mixed thermoplastic polymers into individual polymers/monomers which can be used to replace fossil-based polymers for plastic production. In pyrolysis, carbonaceous waste is thermally decomposed at about 400–600 °C in the absence of air into pyrolysis oils, combustible gases, and carbonaceous solids.
With gasification, carbonaceous waste is converted under high temperature and pressure conditions with reactants (i.e., steam and oxygen) into synthesis gases (mainly carbon monoxide and hydrogen) which can then be further synthesized to (“green”) basic chemical products.
Chemical recycling has significant relevance
Chemical recycling is a potential solution for wastes as mechanical recycling reaches its limits. A scientific review of chemical recycling technologies indicated that particularly pyrolysis and gasification technologies have been reported as being suitable for a range of mixed and challenging carbonaceous waste inputs which are not suitable for mechanical recycling due to contamination, sorting, and technological difficulties.
These include for example end-of-life tires, sewage sludges, municipal solid waste, carbon/glass-fiber reinforced and composite materials as well as hazardous waste. Moreover, R&D cooperation and pilot developments utilizing carbonaceous waste other than plastic waste as chemical recycling input are also observed.
For example, Enerkem as well as the Institute of Energy Process Engineering and Chemical Engineering at the TU Bergakademie Freiberg have investigated several different waste types such as municipal solid waste, agricultural residues, and even ocean waste as feasible feedstock for chemical recycling via their pilot gasification facilities in Canada and Germany respectively. BASF uses tire-derived-pyrolysis-oil (TDO) from end-of-life tires (ELT) as secondary raw material for chemical production, while Audi has initiated a cooperation with the Karlsruhe Institute of Technology for the chemical recycling of automobile composites which cannot be mechanically recycled.
The integration of chemical recycling products such as pyrolysis oil and syngas from gasification as flexible intermediate raw materials to replace fossil-based feedstock to produce a broad spectrum of useful chemical products such as ammonia, olefins and other chemicals is increasingly emphasized by both science and industry.
For example, BASF has assessed the feasibility of using pyrolysis oil as a naphtha substitute in its chemical value chain. Enerkem in Canada converts municipal solid waste (MSW) via gasification into methanol – an important and highly versatile basic chemical building block for the manufacture of a wide range of value-added chemical products.
Additionally, since 2003, Showa Denko in Japan has operated a commercial gasification plant to convert plastic waste into syngas, and subsequently into ammonia and other chemicals.
Chemical recycling’s crucial benefits
A crucial benefit of chemical recycling is resource conservation, with the added benefit of the (high) potential for emissions avoidance and reduction of environmental impacts associated with landfills and incineration. This corresponds to scientific literature which points to the environmental potential of chemical recycling. Significant efficiency and technological advantages over conventional recycling and disposal routes can be seen. This leads to the potential to increase the scope and volume of waste recycling to meet higher recycling quota for the generation of higher-value products without downcycling. It emphasizes the relevance of chemical recycling for the recycling of problematic waste.
Conclusion
Multiple market drivers are awakening interest in chemical recycling as an emerging theme in waste management. Chemical recycling processes span three major components. They use waste as input to produce outputs such as gases, oils and basic chemicals via thermochemical conversion processes which break down the input material into molecules. In comparison to conventional recycling and thermal treatment methods [e.g., incineration as a waste-to-energy (WtE) path*], chemical recycling is associated with significant additional benefits especially in terms of resources conservation, reduced environmental impacts, efficiency, technological advantages, product, and input flexibility.
Given the abundance of the available feedstock (waste), chemical recycling is set to be a game changer. It can close the loop in the value chain and enable its stakeholders to reach the aggressive circularity levels that they have targeted for the next decade.
Source: Roh Pin Lee, Manja Tschoepe, Raoul Voss, Perception of chemical recycling and its role in the transition towards a circular carbon economy: A case study in Germany, Waste Management, Volume 125, 2021, Pages 280-292, ISSN 0956-053X, https://doi.org/10.1016/j.wasman.2021.02.041. ©2021 The Authors. Published by Elsevier Ltd. The source text is an open access article under the CC BY-NC-NDlicense. The term "plastic" in the source text was replaced by the term "carbonaceous waste" and essential additions to the original text in the present summary have been marked with an asterisk *.
Weibold is an international consulting company specializing exclusively in end-of-life tire recycling and pyrolysis. Since 1999, we have helped companies grow and build profitable businesses.









